DEHLI ( WEB NEWS )
The Indian Health Ministry said in a statement that test samples of Coldrif cough syrup found a highly toxic industrial solvent. At least nine children died allegedly after consuming it.
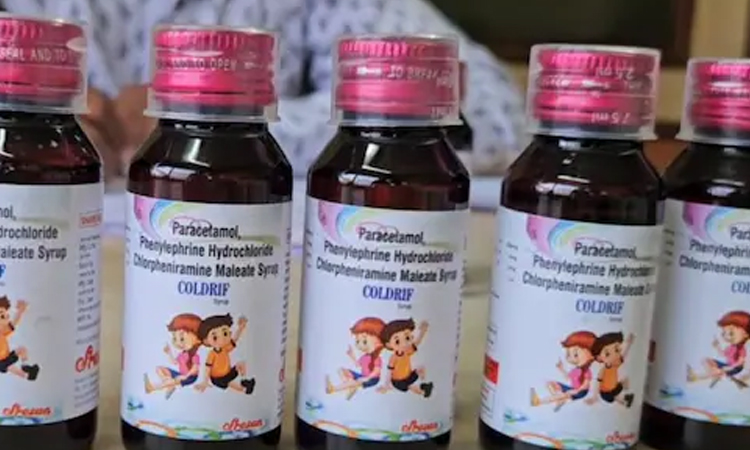
NEW DELHI, Oct 5, 2025 (BSS/AFP) – At least three Indian states have banned a cough syrup after several children died allegedly after consuming the product, said local authorities and reports.
The death of at least nine children, all aged under five, since late August, in the states of Madhya Pradesh and Rajasthan have been linked to a cough medicine they were prescribed.
India’s health ministry Saturday said laboratory tests on samples of the syrup the children had consumed revealed it was contaminated with diethylene glycol (DEG), a toxic substance used in industrial solvents that can be fatal if ingested even in small amounts.
“The samples are found to contain DEG beyond the permissible limit,” the ministry said in a statement.
The product sold under the brand name Coldrif Cough Syrup was manufactured by Sresan Pharma at a unit in the southern state of Tamil Nadu.
“The sale of this syrup has been banned throughout Madhya Pradesh,” said Mohan Yadav, chief minister of the central state of Madhya Pradesh, where most of the deaths have been reported.
“The sale of other products from the company that manufactures the syrup is also being banned.”
Authorities in the states of Tamil Nadu and Kerala have also banned the product, local media reports said.
The Indian Health Ministry said in a statement that tests samples of Coldrif cough syrup, made by Sresan Pharma in Tamil Nadu, contained diethylene glycol (DEG).
“The samples are found to contain DEG beyond the permissible limit,” the ministry statement said.
“The sale of this syrup has been banned throughout Madhya Pradesh,” said Mohan Yadav, the chief minister of the central Indian state, where most of the deaths occurred.
Yadav added that the sale of other products of Sresan Pharma is also being banned.
Tamil Nadu and Kerala have also banned sales, according to local media.
India’s pharmaceutical industry under scrutiny
The incident has renewed scrutiny of India’s pharmaceutical industry. In 2022, the World Health Organization (WHO) linked cough syrups made by another Indian company to the deaths of 70 children in The Gambia.
A year later, the WHO again warned against two cough medicines made in India after Uzbekistan linked the syrup to the deaths of at least 18 children.
A fifth global warning against India-made medication in a 10-month span was issued after medicines contaminated with toxins were found in Iraq.
India provides 20% of the world’s supply of generic medicines, according to a Press Information Bureau (PIB) statement in May.
These are cheaper versions of drugs produced once patents expire.
Its pharmaceutical industry ranks third in the world by volume, the Indian government agency added.
In 2011, a study published by the Indian Journal of Pharmacology found that while generic drugs were five to six times cheaper than their branded equivalents, there was no significant difference in quality.

